2025 Research Coordinator Meeting Recap
Our 3rd RC meeting is a wrap. We appreciate your participation and hope you found the experience valuable—And for those who couldn't make it this time, we missed you and hope to see you at a future event!

Meeting Highlights & Takeaways
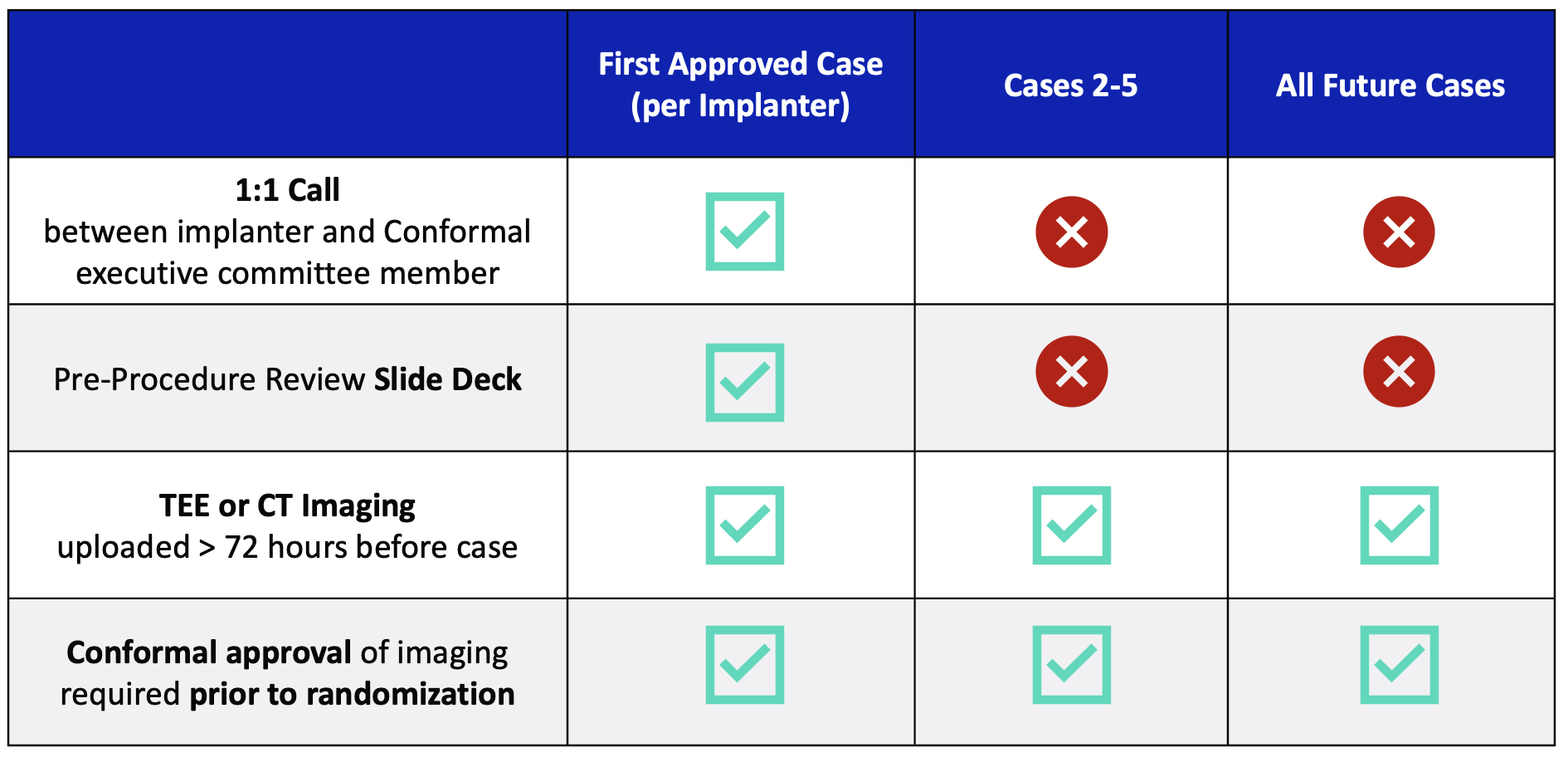
High Level Imaging Requirements
Protocol requires that TEE or CT imaging is reviewed and approved by Conformal PRIOR to randomization. Please upload TEE or CT imaging 72 hours before upcoming case and WAIT TO RANDOMIZE until you get written approval of suitability!
First Case (per implanter):
- 1:1 call required
- Pre-Procedure Review Slide Deck required
- TEE or CT imaging required
- Conformal approval prior to randomization required
Cases 2-5:
- TEE or CT imaging required
- Conformal approval prior to randomization required
All future cases:
- TEE or CT imaging required
- Conformal approval prior to randomization required
Protocol Changes for CONFORM Rev K.
-
-
Screening Imaging:
A CT or TEE is required prior to randomization to properly assess all I/E criteria. Cardiac MRI or TTE alone are not sufficient to randomize the patient. -
-
Pre-Discharge TTE:
Must be done 4+ hours after end of procedure. -
-
Cardiac CT allowed at 45-day follow up:
Findings of leak or thrombus on CT must be confirmed on TEE and resolution must be documented. -
-
Follow-Up Schedule - Attempted Population:
Followed for 18-Months (telehealth only).
RC Panel Takeaways
How to Identify and Engage Subjects
-
Partner with the Clinic Team: Collaborate with site staff and consider referrals to find potential subjects.
-
PI Involvement Matters: The PI should meet with each subject, review device options, and help determine the best fit.
-
Streamline the Process: When possible, schedule consent and imaging on the same day to minimize visits.
-
Early RC Engagement: When possible have the RC in the room during the subject’s first visit to help explain the trial and start building a strong relationship right away.
Follow-Up &
Retention Tips
-
Explain follow-up requirements during screening and why they’re important for the study.
-
Compare CONFORM follow-ups to standard care so patients see they’re similar.
-
If acceptable share your or the PI’s phone number so patients can easily reach out with questions.
-
Build personal connections with your patients—it helps with long-term engagement.
-
Try and be flexible with scheduling, as many subjects may only be available outside regular work hours.
Have a CLAAS AcuFORM Demo Device Ready
-
Keep demo devices available so patients can see and feel the difference—especially the soft, conformable foam in CLAAS AcuFORM.
-
Demo devices help explain things that may be hard to understand with medical terms alone.

Communication &
Connection is Key
We know many of you are juggling multiple clinical trials, and it's not always possible to remember every detail. That’s why staying connected matters. One of the greatest strengths of the CONFORM RC community is the knowledge and experience you all bring—and share.
We hope this meeting helped you make new connections and sparked conversations that continue beyond the meeting. Let’s keep communicating, supporting each other, and sharing the tips, tricks, and insights that drive our collective success.
Together, we’re stronger.
Additional CONFORM Trial Resources
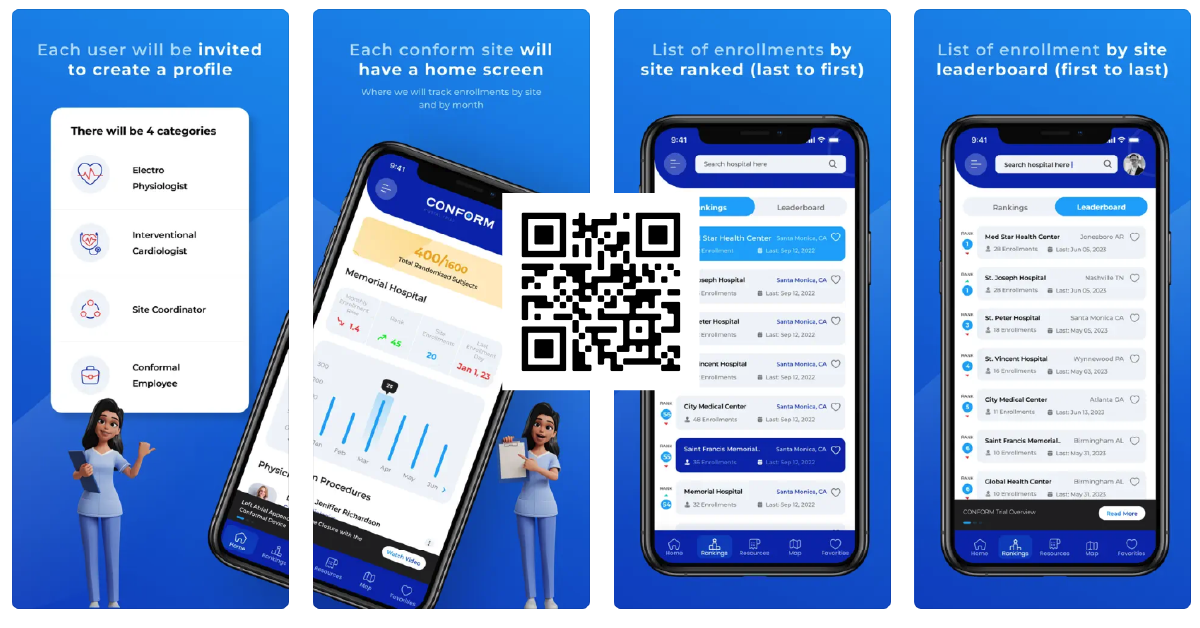
Download the CONFORM APP
A mobile solution for physicians and coordinators to monitor and manage thier patient enrollment activities.
CONFORM Trial Patient Site
A new site to help inform and educate prospective patients seeking an alternative to long-term blood thinners.
Thank you CONFORM Research Coordinators! We can't do this without your support.

