Contact Your CONFORM Trial Site Manager
Our Clinical Operations Team is here for you. We ensure the smooth coordination and execution of clinical trials and provide support and guidance. Whether you have questions, need assistance with protocols, or require any other support related to the CONFORM Study, our team is here to help. Use the links below to email your site manager.
Chad Badorek
Sr. Manager Clinical Operations
Pam Phongpharnich
Sr. Clinical Research Manager
Kelly Kwong
Sr. Clinical Research Associate
Melissa Ricketts
Sr. Clinical Research Associate
Elizabeth Raskulinecz
Sr. Clinical Research Associate
Carmalee Estell
Sr. Clinical Research Associate
Briana Knapp
Sr. Clinical Research Associate
Cassandra Oluwayomi
Sr. Clinical Research Associate
Janvi Shah
Sr. Clinical Research Associate
Kurt Lang
Clinical Research Associate II
Contact Your CONFORM Trial Site Monitors
Sangnyui “Sang” Tani
Sr. Clinical Research Associate
Charlie Diep
Sr. Clinical Research Associate
Joyce Alston
Sr. Clinical Research Associate
Tu Tran
Sr. Clinical Research Associate
Lauren DiGiacomo
Sr. Clinical Research Associate
CONFORM Pivotal Trial Online Resources

Conformalmedical.com
Learn about Conformal Medical, the CLAAS® System, and the CONFORM Clinical Trial.
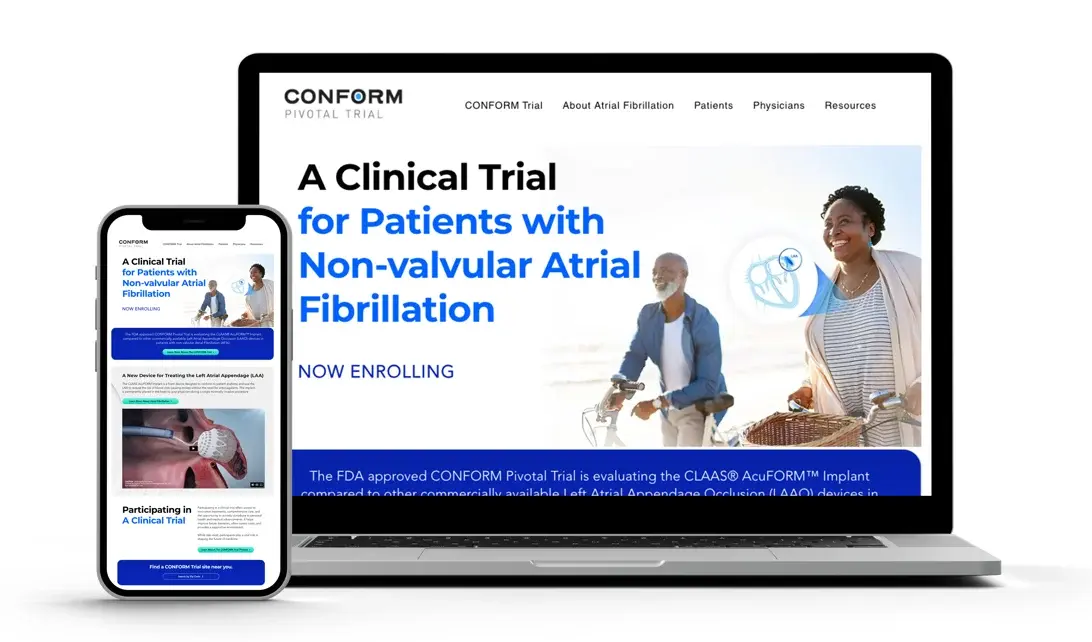
CONFORMtrial.com
A new site to help inform and educate prospective patients seeking an alternative to long-term blood thinners for stroke risk reduction in non-valvular atrial fibrillation.
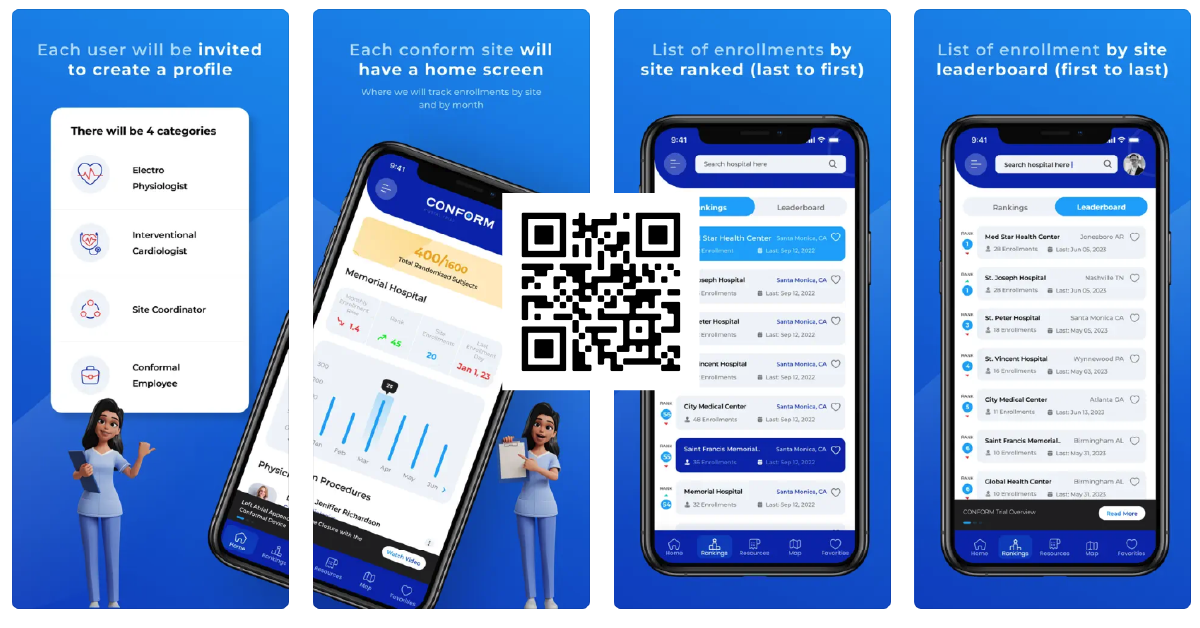
Download The CONFORM Trial App
The app provides real-time updates on patient enrollment status, allowing hospitals to quickly identify and address any issues or delays in the process. Additionally, the app generates reports and analytics to give hospitals valuable insights into their enrollment performance and identify areas for improvement.
